

Metal reactivity table series#
Apart from this, the reactivity series also leads to a single displacement reaction, which involves the reduction of ions from the low reactive metals because of the high reactivity metals.Also, to predict the reaction between the metals and acids, and their end products, using the reactivity series.Besides, to release the hydrogen gas from the cold water, the metals from the reactivity series can be employed.In addition to this, to displace low reactivity metals from the high metal salts, reactivity series can be used.
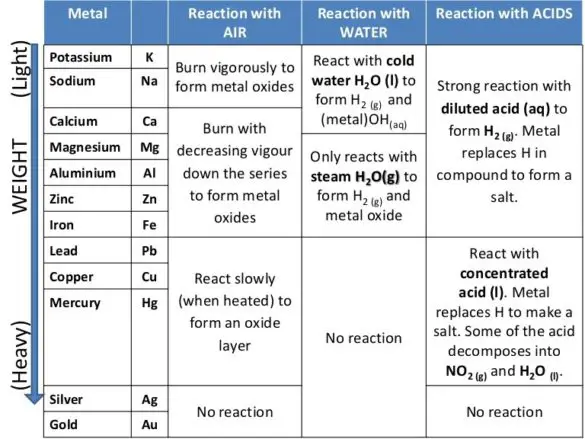
Firstly, the reactivity series is actively employed in the extraction of metals.As you can understand from the above sections, reactivity series is a very important concept in Chemistry, and so, it has various applications, which we will talk about briefly below.Furthermore, when metals from the reactivity series come into contact with sulfuric acid or HCL, then they will extract hydrogen ions from these diluted solutions.Moreover, when we go down the metal reactivity series, we can also find out that the unique property of elements that allow them to separate hydrogen from the hydrides decreases.For instance, the top metals on the list can easily remove the low metals in the series from their salts. Besides this, the most reactive metals in the series have the unique property of eliminating less reactive elements from their own salts.Along with this, the electron-donating capacity of metals down this series also decreases.In present metals at the top of the reactivity series, high electro-positivity is observed, which decreases when going down the series.Now that we have understood what reactivity series is? Let us now focus on learning the various features of the reactivity series.The reaction is limited to a small number of strong oxidizing acids. Strong reaction to acids, poor reaction with steam Slow reaction to cold water, fast reaction to hot water, vigorous reaction to acids. Here in the below section, we will list out the metals, along with their reactive substances. Reactivity of Metals with Various SubstancesĪs a part of the metal reactivity series, certain elements only react with certain substances, while some can react with two or more substances. Thus, with very small changes, the atoms in the elements tend to lose electrons quickly. So, metals that have high atomic numbers will be more reactive and will be prone to losing electrons easily. The first thing you have to remember is that the reactivity is only observed in metals and not in any other elements that are present in the periodic table. Furthermore, reactivity is also known as activity series, and the main reason why this phenomenon is actively found in metals is that the outer orbitals in these elements are incomplete.
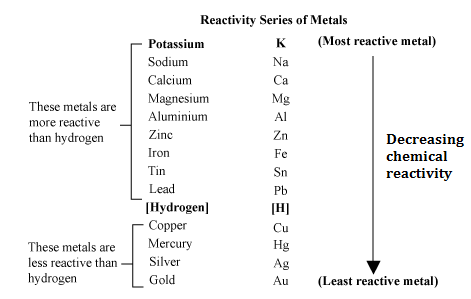
The reactivity series is a periodic trend that explains how the reactivity of various elements decreases or increases from horizontal to vertical in the modern periodic table. This helps you understand the reactivity series in more detail. Do you know the reactivity of copper or aluminium? Do you know which element reacts with which substance?Īnswers to these questions can be found easily with the help of the reactivity series, which will explain how the element’s reactivity varies with respect to its group, atomic number, and mass.


 0 kommentar(er)
0 kommentar(er)
